Trendgineering
High precision injection molding for inhalers
| Petra Rehmet
Complex mechanics place high demands on technology
They are small and often fit in any pocket. Inhalers, which make life easier for asthma patients and other chronically ill people, often have complex mechanics inside. The production of these filigree components requires a particularly high degree of precision and freedom from burrs in the process. Requirements that KraussMaffei meets. Thomas Hörl, Head of Expert Sales Medical at KraussMaffei, and Joachim Köbelin, Expert Medical Technology, explain the special challenges of medical technology manufacturing.
ahead
Inhalers are available on the market in a wide variety of forms and functions. How are they best distinguished?
Joachim Köbelin
Inhalers are basically differentiated into four different types: metered-dose inhalers, dry-powder inhalers, soft mist inhalers and nebulizers as well as electric inhaler devices. Each system has its specific advantages and disadvantages and is prescribed based on medication as well as the patient's fitness. Metered-dose inhalers, for example, are compact, easy to carry with you and can be used without any special preparation. They enable high and uniform amounts of the desired dose to be released, regardless of the user's breathing efficiency. This is an advantage.
The duration of inhalation is short. However, slowly inhaling and simultaneously triggering the spray requires a certain amount of coordination, which is not easy for every patient. In addition, the device should be shaken each time before it is triggered to prevent dosing errors due to sedimentation of the active ingredient particles.

Different systems require different breathing techniques
A selection of different inhalers on the market
ahead
Metered-dose inhalers are often operated using propellant. That doesn't sound particularly environmentally friendly. Are there alternatives?
Thomas Hörl
The dry-powder inhalers work without propellant. They are triggered by the user taking a breath, so, compared to a metered-dose inhaler, only a small amount of coordination is required of the patient. However, release of the dose depends directly on the inspiratory airflow. In other words, a breathing efficiency of 30 l/min. is required. This could be problematic in the case of acute dyspnea, severe illness or with small children.
ahead
How exactly do these dry-powder inhalers work?
Joachim Köbelin
A typical example of a dry-powder inhaler is Diskus®, which was developed by Gregor Anderson, Head of the Device Technology Group (DTG) at GlaxoSmithKline. Diskus® has been successfully produced on KraussMaffei machines for over 20 years. It consists of a two-piece housing made of polypropylene, a mouthpiece and a lever. On the inside there is a sophisticated mechanical system that consists of numerous intricate, highly functional components produced using injection molding. For us as a provider of complete injection molding solutions this is, of course, a particularly exciting task.
ahead
What is vital in the injection molding process? Are there components of the dry-powder inhaler that have to achieve a rather large amount?
Thomas Hörl
All components are produced on individual injection molding machines and then assembled. Above all this requires high precision, strict adherence to tolerances and a complete absence of burrs. Injection molding production usually takes place in cleanroom conditions of Class 8 or higher. Here, too, KraussMaffei has many years of experience and has already implemented production cells for vaccine containers under cleanroom class 5 in accordance with DIN EN ISO 14644 (or GMP class A) two decades ago.
"The production of the individual components of the dry-powder inhaler requires high precision, strict adherence to tolerances and absolute absende of burrs."Thomas Hörl, Head of Expert Sales & KeyAccount Management MEDICAL
Two components of Diskus® that, for example, have to achieve a lot, are the so-called drug exit port and the mouthpiece. Both are high-precision components. It is important for the powder to remain dry and be atomized as best possible. Drilling the drug exit port is a painstaking trial the requirements for absence of burrs, precision and tolerances are enormous here. It is unthinkable for a component that comes into direct contact with the patient's mouth to have any particle formation or contamination from fines created during take-out automation that would be inhaled.
ahead
Which injection molding machines are suitable for production — hydraulic or all-electric?
Thomas Hörl
In principle both, so the hydraulic CX series or GX series and the all-electric PX series from KraussMaffei. As of late, it is largely all-electric machines that are being used due to their low energy consumption, low heat emission in cleanrooms and reduced noise pollution. Depending on the number of units and size of the components, the number of cavities varies between 8, 16, 32 and 48. Not only single-cavity molds are used, but also multi-cavity molds, such as stack molds on our TwinForm GX machine with two injection units.
ahead
You broached the topic of quality assurance. How important is it for this to include networking, for example, of injection molding machines, automation and peripherals?
Joachim Köbelin
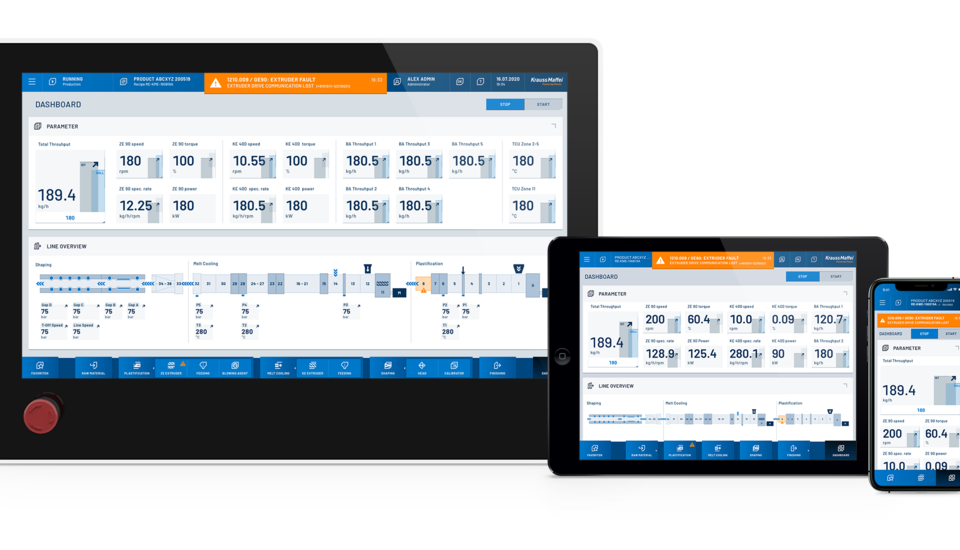
Quality control and seamless documentation are essential. That already takes place at the injection molding machine as part of the process and tolerance monitoring and ensures high reproducibility as part of the rigorous validation phase.
If an injection molding machine, automation and peripherals, such as tempering units and hotrunner controllers, are integrated into just one control system, this is quite clearly an advantage. At KraussMaffei, we achieve this reliably and securely with the MC6 control system, not many systems on the market are capable of this. For example, a data record with all of the quality-related functions and parameters — from the start-up process and tolerance monitoring of all integrated systems to depositing good parts in the packaging box as well as discharging start-up parts or reject parts.
ahead
Let's look ahead to the future. Where do you see the greatest growth potential for inhalers?
Thomas Hörl
The world is changing. Environmental pollution, smog, increasing allergies and respiratory illnesses triggered by these factors, or the as yet unforeseeable long-term effects of Covid-19 will unfortunately subject our healthcare systems to further challenges. We assume that the demand for inhalers will continue to increase in the coming years. Studies show growth rates of seven percent and more. As an experienced system provider of injection molding technology, automation and digital solutions, we are in a strong position here and offer our customers clear added value.
Contact
thomas.hoerl@kraussmaffei.com