Trendgineering
Micro-injection molding at its best with LSR
| Petra Rehmet
PX 25 CleanForm in the cleanroom
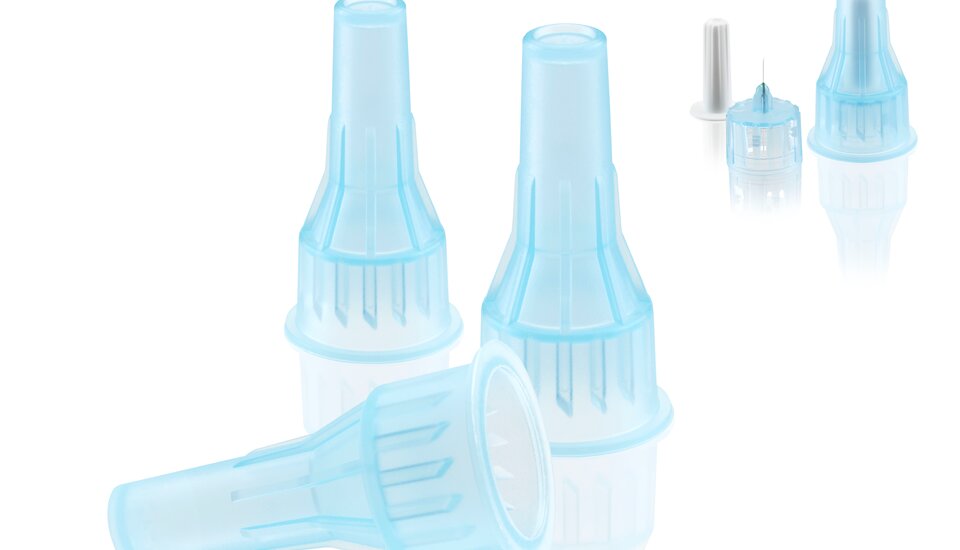
Flexible, temperature-resistant and even bacterially resistant—silicone provides great potential for medical technology and life science applications. At the K, a PX 25 CleanForm produced micro-membranes made of LSR under cleanroom conditions. Cordula Wieland, Product and Technology Manager, explains the special features of this intricate production.
ahead:
Tiny membranes made of liquid silicone, and what's more, this is for the medical industry. That makes the requirements for a manufacturing cell very demanding, doesn't it?
Cordula Wieland:
These are challenges like the ones our customers face on a daily basis. We, of course, want to do our best to support them in this and, accordingly, have designed this project for our smallest electric PX25 machine.
ahead:
Micro-injection molding with integrated automation under cleanroom conditions. What was most difficult?
Cordula Wieland:
The cleanroom conditions are relatively easy to achieve because the machine design of the PX is already meant for clean manufacturing. You only need a couple additional equipment options such as flow boxes. The modular construction set of the PX series provides the necessary flexibility for this. Here, together with our partners, we offer everything needed to create a genuine class 7 cleanroom.
ahead:
And how tricky is the component?
Cordula Wieland:
It weighs only 0.0375 g, so even with eight cavities we have a very low shot weight. That is why we developed an entirely new screw with a diameter of only 12 mm that provides for perfect homogenization of the LSR components. In addition to the screw, it is also necessary to have an intricate nonreturn valve that closes with precision and is rugged.
ahead:
Do such filigree components require particularly handling?
Cordula Wieland:
Yes, the automation was something for specialists. These micro parts are difficult to grip. Usually, standard systems are working with brush-out solutions. However, this does not allow a separation and controlled guidance of the component. In addition, the removal unit should simultaneously have a function for slitting the articles in parallel and precisely with eight very small knives. Fortunately, our automation division is familiar with such challenges and was able to solve these tasks.

In an interview with ahead:
Cordula Wieland
ahead:
A part weight of 0.375 g leaves little room for deviations, right?
Cordula Wieland:
We are helped here by the APC plus machine function. Particularly when working with silicone, you have to expect significant batch fluctuations, which can then lead to overfilling or underfilling. APC plus adjusts the changeover point from shot to shot and thus causes the weight to remain extremely uniform. Even if there are interruptions in production, start-up is very easy. Another advantage: There is clean demolding through automation starting from the first shot, which is usually possible only with great difficulty, particularly for such small components.
ahead:
In medical technology, traceability is everything. How can you ensure this?
Cordula Wieland:
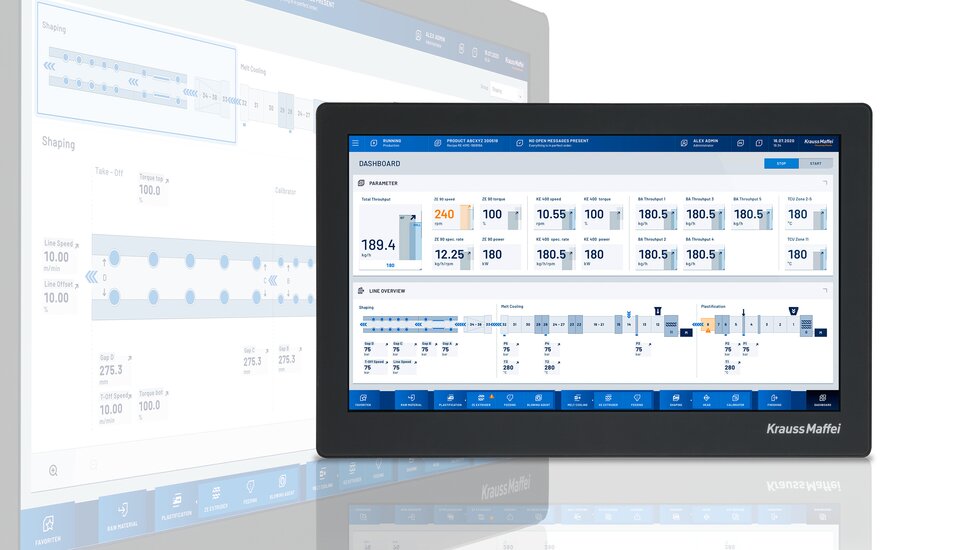
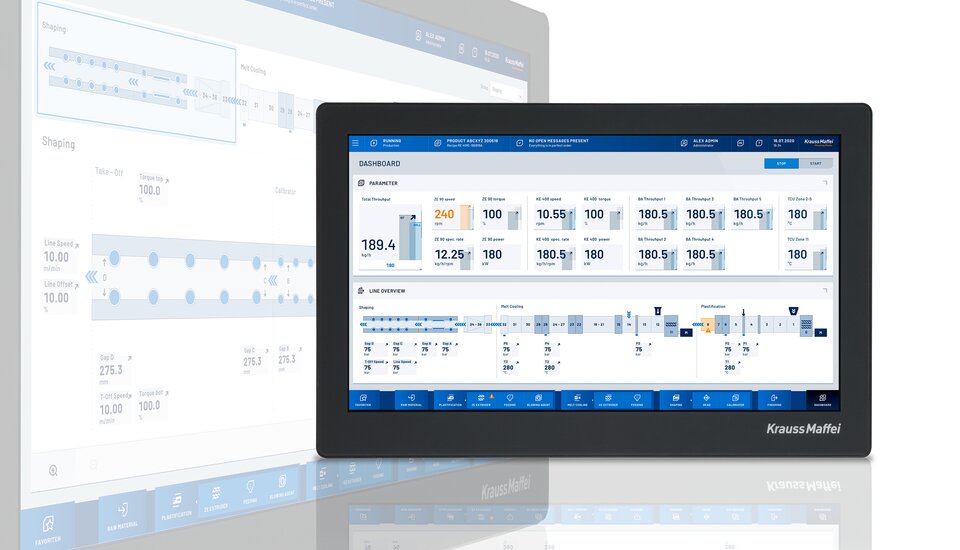
We have in-house specialists for this in the Digital Service Solutions business unit. For example, our dataXplorer data analysis tool can save up to 500 signals per second as continuous curves and thereby document the manufacturing sequences comprehensively. It is like a magnifying glass for the entire process. The automation for this project also includes packaging of the membranes in bags. A QR code is printed on this, which then leads to the individualized process data for the respective articles.
ahead:
Are there actually any new developments in the area of silicone processing?
Cordula Wieland:
Yes, on the whole we have a generation shift in the materials and even new properties, such as self-healing silicone. The new types place even more stringent requirements on the machine and processing competence. In this respect, the area remains very exciting—and we are glad to be leading the way.